The research team directed by Prof. Erwei Song and Shicheng Su at Sun Yat-sen Memorial Hospital revealed a new mechanism of tumor immune evasion regulated by an lncRNA
Source: Sun Yat-sen Memorial Hospital
Written by: Di Huang
Edited by: Wang Dongmei
The research team directed by Prof. Erwei Song and Shicheng Su at Sun Yat-sen Memorial Hospital of Sun Yat-sen University recently revealed a long non-coding RNA termed NKILA promotes the apoptosis of tumor-specific T cells induced by tumor cells, leading to tumor immune evasion, which was published in Nature Immunology on September 17th, 2018. Moreover, the research suggests that engineering lncRNAs in adoptively transferred T cells might provide a novel antitumor immunotherapy.

Breast Tumor Center in Sun Yat-sen Memorial Hospital
Cancer immunotherapy was picked as the outstanding scientific achievement by Science’s top 10 breakthroughs of year 2013 and 2017, which has been proven effective against hard-to-treat lymphoma and melanoma. However, immunotherapy is less effective in breast and lung cancer, suggesting that the underlying mechanisms need further investigation.
Here, the researchers found that tumor-specific T cells were sensitive to tumor-mediated apoptosis as a result of the high expression of NKILA, an NF-κB-interacting lncRNA. In resting T cells, NKILA remains low level. Once T cells were activated to be tumor-specific CTLs, NKILA was up-regulated significantly to inhibit NF-κB activity, which determined the survival of T cells. As a result, T cells were sensitive to tumor-mediated apoptosis, leading to strong suppression of their tumoricidal effects.
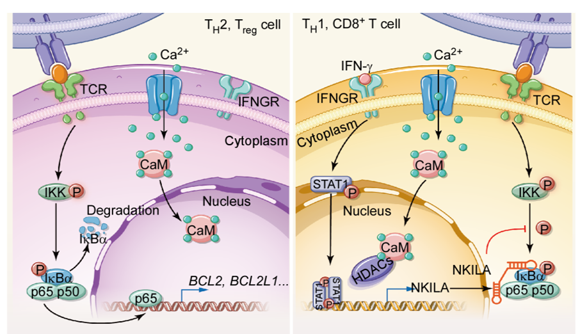
The scheme of the mechanism underlying NKILA expression and contribution to the sensitivity to tumor-mediated apoptosis of different T cell subsets.
More importantly, adoptively transferred CTLs with NKILA knockdown, which sustained the activities of NF-κB, effectively inhibited the growth of breast cancer patient-derived xenografts in mice by increasing CTL infiltration and enhancing their anti-tumor immunity.
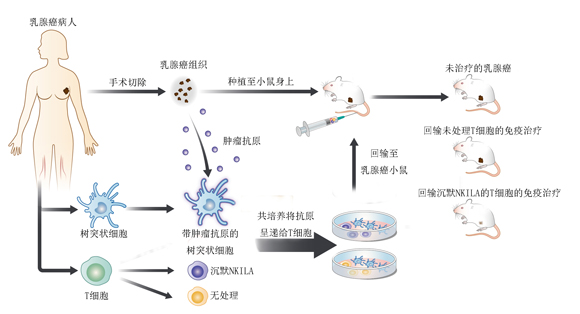
NKILA silencing improves adoptive T cell therapy in PDX breast cancer models.
This work titled ‘NKILA LncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death’ has been published online on September 17th in Nature Immunology.
Prof. Song said they are planning a phase I clinical trial which means the preliminary evaluation of clinical pharmacology and human safety, relying on the platform of the immunotherapy center of Sun Yat-sen Memorial Hospital. “Based on the previous results, we will knockdown NKILA in T cells ex vivo and transfer the engineered T cells to cancer patients. We are confident that silencing NKILA in adoptively transferred T cells can enhance the effects of immunotherapy, which was safer and more reliable than tumor cell gene therapy.
Introduction to Professor Erwei Song:
Professor Erwei Song is President of Sun Yat-sen Memorial Hospital, Sun Yat-sen University.
Prof. Song has focused his research work on the metastasis of malignant tumors, mainly on breast cancers, and got a series of novel discoveries in unraveling the mechanisms of cancer cell plasticity and metastasis regulated by non-coding RNAs (ncRNAs) and tumor microenvironment: 1) His work demonstrates the important roles of multiple microRNAs in regulating breast cancer biology, including stem-cell like feature, epithelial-mesenchymal transition, invasiveness, metastasis and therapeutic sensitivity, which are related to breast cancer progression and treatment response in clinics; 2) His research found that Long non-coding RNAs(lncRNAs) could participate in the inflammatory pathway in tumor as a signal transducer, his research on NKILA revealed a new interaction mechanism between lncRNAs and protein; 3) His research work on tumor microenvironment identified a novel functional receptor for macrophage specific CCL18 that plays an important role in promoting breast cancer metastasis and his further work demonstrated a positive feedback loop between breast cancer cells and TAMs in promoting cancer metastasis. In addition, his recent work identified a novel cancer associated-fiborblasts subset (CD10+GPR77+CAFs) that could sustain cancer stemmess and promote tumor chemoresistance, which was relied on the sustaining NF-?B activation in this CAFs subset induced by complement signaling. Altogether, he has published 105 articles in SCI-indexed journals, 62 among which he was the first author or the corresponding author. These journals include Cell, Cancer Cell, Nature Immunology, Nature Medicine, Nature Biotechnology, Science Translational Medicine, etc. The total SCI citation frequency is more than 5000 so far. As a corresponding author, one of his articles has been cited 1,097 times.
His achievements obtained wide attention and highly recognized by international counterparts. His researches were selected as representative of the “Ten Breakthrough of the year 2003” by Science, and won a place among the “Top Ten Progresses in Science and Technology in Academic Institutions in China” by the Ministry of Education in 2008. He further won the first prize of the “National Natural Science Award by the Ministry of Education of China” in 2012, the “Ho Leung Ho Lee Foundation Award” in 2013, and notably, the second prize of the "National Natural Science Award" by the State Council in 2015. The “Spotlight on China” (supplement to Cancer Cell) introduced his research work on a special issue.
Link to the paper: https://www.nature.com/articles/s41590-018-0207-y
