Research team directed by Prof. Limin Zheng from School of Life Sciences made progress in deciphering the myeloid immune contexture in hepatocellular carcinoma
Source: School of Life Sciences
Written by: School of Life Sciences
Edited by: Tan Rongyu, Wang Dongmei
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide, with an increasing incidence and a dismal prognosis. In contrast to most other malignancies, 90% of HCCs arise from chronic inflammation. Although the tumor microenvironment is supposed to be the battle ground for host immune cells to eliminate cancer, the tumor usually “educates” its neighbor cells to convert the hostile microenvironment to a promoting milieu for tumor progression. Myeloid cells, including monocytes/macrophages, polymorphonuclear leukocytes, myeloid-derived suppressor cells and dendritic cells, are a group of heterogeneous innate immune cells that constitute a major component of the tumor tissue and are key regulators of the immune milieu. Although our knowledge of the tumor-associated myeloid cells has substantially increased over the past decades, challenges remain in interpreting the myeloid response in the microenvironment and translating this information into clinical benefits.
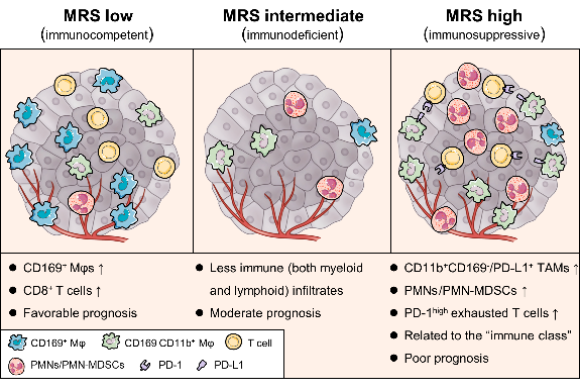
Prof. Limin Zheng and colleagues recently screened 18 myeloid features that cover major myeloid subtypes in human tumors and then constructed and validated a simple prognostic myeloid signature, called the myeloid response score (MRS), based on a dataset from a multicenter cohort of >1,100 HCC patients. Combining immunological, molecular, and bioinformatic investigations, this study indicates that the MRS is able to describe the immune microenvironment of HCC and thereby predict disease prognosis. HCCs with different MRSs not only differ in the intratumoral myeloid contexture but also show distinct cytotoxic T lymphocyte activation/exhaustion statuses. In this large, multicenter cohort, the MRS as a simple and reliable scoring signature is superior to the current staging systems in predicting postsurgery HCC prognosis, exhibiting notable discriminatory ability, accuracy, and clinical usefulness. Additional evidence suggests that the MRS may also be associated with the therapeutic efficacies of a fist-line targeted drug and immunotherapies for HCC.
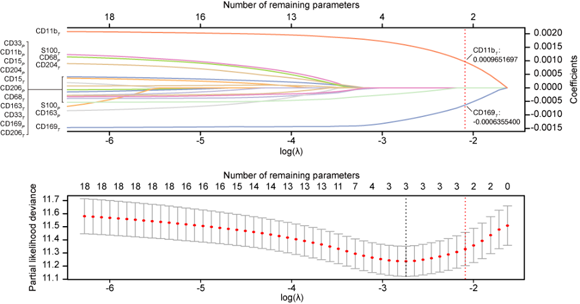
Collectively, the MRS can serve as a measure to assess the balance of the microenvironmental myeloid response to predict disease and therapeutic outcomes. This novel myeloid signature may help to establish the stratification of HCC immune subtypes and may aid in a wide range of clinical decisions and tailoring individual treatment options.
This work entitled "Myeloid signature reveals immune contexture and predicts the prognosis of hepatocellular carcinoma" was published in the prestigious medical journal
The Journal of Clinical Investigation (H-index: 471) on Sept. 1st, 2020. Prof. Limin Zheng is the corresponding author. Dr. Chong Wu, an associate research fellow, and Jie Lin, a postgraduate are co-first authors. This research, together with the team’s previous work published in the same journal in 2018 (
J Clin Invest, 2018. 128(8): 3425-38) fist-authored by Dr. Chong Wu, provides important novel insights into the origin and balance of the tumor-promoting systemic myeloid response. This research was financially supported by the National Key R&D Program of China, the National Natural Science Foundation of China, and the Fundamental Research Funds for the Central Universities.
Link to the paper:
https://www.jci.org/articles/view/135048