The collaborative research between Prof. Boxing Li at Zhongshan School of Medicine and Prof. Richard W. Tsien at New York University has recently been published in Cell
Source: Zhongshan School of Medicine
Written by: Zhongshan School of Medicine
Edited by: Tan Rongyu, Wang Dongmei
Neurons are highly plastic, allowing our brain to adapt to its environment. Homeostatic plasticity, one of the key features of the neurons, helps to control neuronal activity by avoiding extremes and allowing network stability. Homeostatic plasticity plays a pivotal role in the regulation of a variety of important physiological functions. For example, during the sleep/wake cycle, homeostatic plasticity helps to regulate the structure and function of synapses and maintains neuronal network stability. Abnormal homeostatic plasticity is related to schizophrenia, epilepsy, autism and neurodegenerative diseases. Homeostatic plasticity of neuronal intrinsic excitability is important in stabilizing the activity of neuronal circuitry, but the underlying mechanisms are not known.
Recently, Professor Boxing Li and his research group at Zhongshan School of Medicine, Sun Yat-sen University, in collaboration with Professor Richard W. Tsien’s group at New York University, discovered the molecular mechanisms of homeostatic plasticity of intrinsic excitability.
They used sodium channel blockers (TTX) to block action potentials to induce the long-term decrease in neuronal excitability. After TTX withdrawal, the duration of the action potential was significantly prolonged, suggesting that neurons exhibited homeostatic plasticity in intrinsic excitability. Further mechanistic studies showed that the homeostatic regulation was due to the reduction of Nova-2-mediated alternative splicing of potassium channel (BK channel) mRNA.
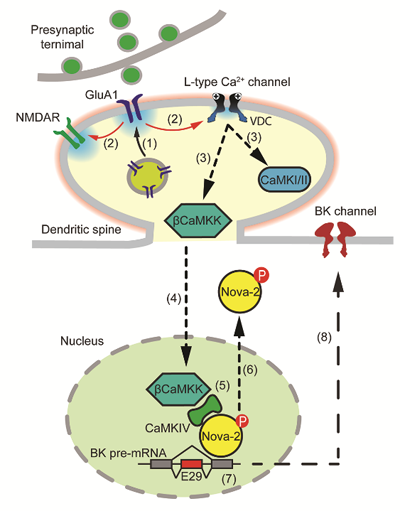
Figure 1. Diagram of the signaling pathway of homeostatic plasticity in intrinsic excitability
It is worth noting that they found that when neurons were treated with TTX for a long period, although the cell body of the neurons did not generate action potentials, the synapse produced significant depolarization, which was sufficient to activate the L-type calcium channel at the synapse. The latter conveyed information into the nucleus through its downstream calmodulin kinases (βCaMKK and CaMKIV), causing Nova-2 phosphorylation and migration out of the nucleus, resulting in a decrease in alternative splicing of BK channel mRNA (Figure 1).
Figure 2. The autoregulatory feedback loop of neuronal excitability
The study provided complete evidence for the “autoregulatory feedback loop” proposed nearly 30 years ago (Figure 2). Multiple molecules in this pathway (AMPA receptor, L-type calcium channel, calmodulin kinase family, Nova-2, BK channel) are closely related to neuropsychiatric diseases such as autism, schizophrenia, depression. Thus, it is implied that the abnormality of this signaling pathway may be an important mechanism for the etiology of the above diseases.
This work was recently published online in
Cell, entitled “Neuronal inactivity co-opts LTP machinery to drive potassium channel splicing and homeostatic spike widening”. Prof. Boxing Li and Prof. Richard W. Tsien are the corresponding authors. Prof. Lianyan Huang and members in Li's Group (Zhengyi Luo, Chuanchuan Wei, and Sikun You) made significant contributions to this work. This work was supported by the National Key R&D Program of Chin, National Natural Science Foundation of China, Guangdong Natural Science Foundation and Guangdong Provincial Key R&D Programs.
Link to the paper:
https://doi.org/10.1016/j.cell.2020.05.013
