
Source: School of Materials Science and Engineering
Written by: Lin Zhaoyong
Edited by: Wang Dongmei
The research group of Prof. Guowei Yang from School of Materials Science and Engineering at Sun Yat-sen University published a paper on the progress in energy nano-materials in Nature Communications on October 2nd, 2018.
Solar-driven photocatalytic H2 evolution through water splitting is a new energy and environmental technology that directly turns low-density solar energy into high-density chemical energy for the future hydrogen economy. Photocatalysts have so far focused on crystals since Fujishima and Honda from Japan realized water splitting over a TiO2 single crystal in 1972, whereas amorphous materials are usually evaluated to be photocatalytically inactive. The self-trapping effect of tail states hindering the carrier transport is considered as the root of failure. However, the research group of Prof. Yang has already pointed out the important application of amorphous materials in electrochemistry thanks to their abundant active sites. For example, the group has demonstrated that using amorphous materials as the electrode materials for supercapacitors, the electrochemical performance is comparable to that of the crystalline counterparts (Nature Communications 4 (2013) 1894). These findings not only correct the previous improper viewpoint on the electrochemical behavior of amorphous materials, but also open a door to the application of amorphous materials in new energy devices. Considering that photocatalysis is actually an electrochemical process, the group theoretically believes that amorphous materials may also realize efficient photocatalytic H2 evolution through water splitting.
Recently, the research group of Prof. Yang has made progress in amorphous photocatalysts. They have, for the first times, proposed and demonstrated that photocatalytically inactive amorphous materials can be transformed into efficient photocatalysts for H2 evolution through two-dimensional confinement (2DC), i.e., 2D amorphous photocatalysts. They adopted their developed metal oxides amorphization technology of laser ablation in liquids (LAL) to obtain 2D amorphous NiO nanoflakes from the suspension of NiO crystals in pure water, and demonstrated they can realize efficient photocatalytic H2 evolution without any noble metal cocatalysts. Further, based on a lot of experimental data and theoretical analysis, they proposed the working mechanism of 2D amorphous photocatalysts, i.e., 2DC (see Figure 1). The long-range disorder and short-range order of the amorphous structure as well as the 2D effect result in electron doping to compensate the intrinsic hole doping in NiO and overcomes the hole localization of crystalline NiO. Meanwhile, the 2D structure shortens the carrier transport distance, and the photo-generated carriers can transport to the surface of 2D amorphous NiO nanoflakes effectively. The amorphization-induced abundant coordination defects and the 2D structure-induced increased specific surface areas provide a mass of active sites for the following redox reactions. These processes endow 2D amorphous NiO nanoflakes with the efficient photocatalytic activity and favorable stability. Obviously, this work not only opens the way for amorphous materials as efficient and low-cost photocatalysts in solar-driven photocatalytic H2 evolution through water splitting, but also enriches the family of photocatalytic materials.
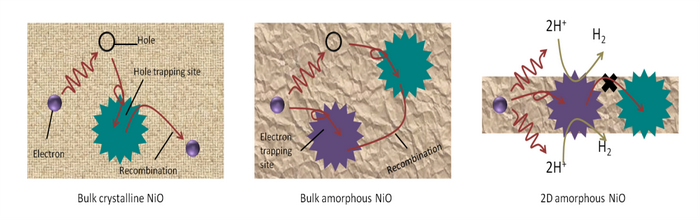
Figure 1. The working mechanism of 2D amorphous photocatalysts, i.e., 2DC.
Further, surface plasmon resonance (SPR) can be introduced by the addition of methanol in the LAL amorphization process to achieve H doping and the resulting electron doping. The position and intensity of the SPR peak can be adjusted by the concentration of methanol solution. Actually, 2D amorphous plasmonic NiO nanoflake is a photocatalyst with an incorporate antenna-reactor plasmonic structure, which can realize two-pathway photocatalysis, i.e., interband transition upon ultraviolet irradiation and plamonic antenna effect upon visible light irradiation (see Figure 2). The photocatalytic activity can be boosted greatly by a factor of nearly 20.
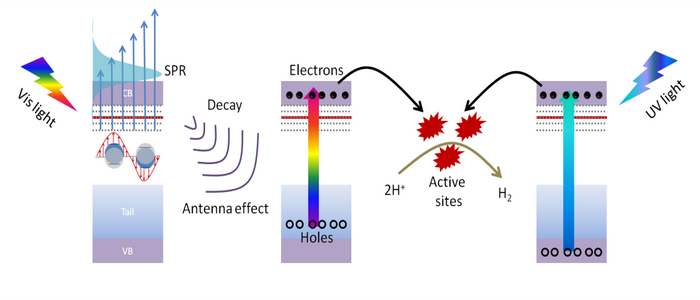
Figure 2. The working mechanism of the incorporate antenna-reactor plasmonic structure.
This work has been reported as a paper entitled “Two-dimensional amorphous NiO as a plasmonic photocatalyst for solar H2 evolution” in Nature Communications (Nature Communications 9 (2018) 4036), which is accomplished independently by Prof. Guowei Yang’s research group. Dr. Zhaoyong Lin is the first author, and Prof. Guowei Yang is the corresponding author. This work is supported by The National Basic Research Program of China (2014CB931700) and State Key Laboratory of Optoelectronic Materials and Technologies.
Link to the paper: https://www.nature.com/articles/s41467-018-06456-y