Prof. Barboiu from the Lehn Institute of Functional Materials developed adaptive hydroxy channels for selective water cluster permeation
Source: School of Chemistry
Edited by: Tan Rongyu, Wang Dongmei
Proteins have evolved for millions of years to adopt their current functional structures. Although they are remarkable in functional efficacy, their structural complexity and environmental sensitivity undermine their reproducibility and further applications for “out of membrane cell” use. Therefore, one of the most ambitious goals in current separation science and technology is to accomplish the reconstruction of natural carriers or channels through synthetic design.
Aquaporins are widespread natural proteins in living organisms that form channels spanning cell membranes to control the translocation of water, while rejecting all ions. Accordingly, artificial water channels (AWCs) have been studied for many years to achieve water permeability very close to their natural counterparts, fulfilling the transport of water across membranes. As shown in Fig. 1, a decade after their discovery, the following AWCs have been found and well investigated, including carbon nanotube porins (CNTPs; Fig. 1a,b), Pillar[5]arene (PAP5; Fig. 1c), Pillar[4]arene (PAH[4]) clusters (Fig. 1d), Aquafoldamers (Fig. 1e), Porous organic cages (POCs; Fig. 1f), Pillar[5]arene hydrazones (PAH5; Fig. 1g), Pillar[5]arene-AQPs (Fig. 1h) and I-quartet water channels (Fig. 1i,j), of which only a few synthetic channels are capable of selective water transport. Hence, only through intelligent molecular design, the functions of aquaporins can be mimicked by AWCs. Moreover, synthetic channels have several advantages over natural proteins, such as cost-effective, chemically robust and easy compatibility with membranes, which make them particularly promising AWCs candidates for industrial applications.
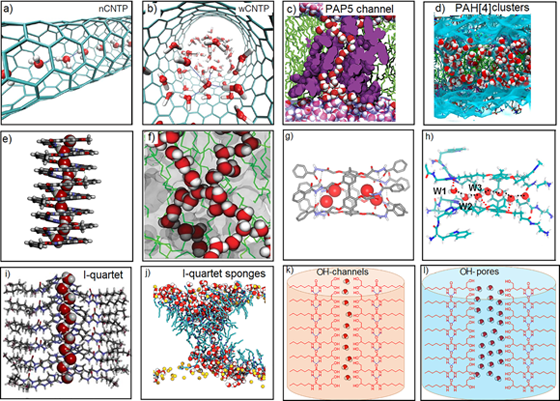
Fig.1 Structures of well-investigated artificial water channels.
Recently, Prof. Mihail Barboiu and his PhD student, Li-Bo Huang, from Lehn Institute of Functional Materials (LIFM), School of Chemistry, Sun Yat-sen University developed adaptive hydroxy channels for selective water cluster permeation. Such OH-channels also achieve the rejection of almost all ions and even protons. Their structures are shown in Fig. 2. The octyl acts as the "tail" of the molecule, which allows the channel to be steadily embedded into the lipid bilayer; the ureido acts as the "backbone" of the channel, and the continuous and iterative H-bonding plays a vital role in their stabilization; the polyols act as the "head" of the molecule, which contact with H
2O directly in order to transport water and reject ions by controlling the size of the channel.
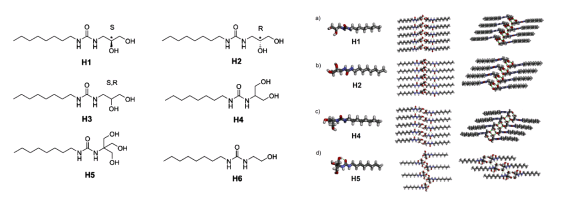
Fig. 2. Chemical (left) and crystal (right) structures of the compounds for hydroxy channels.
Furthermore, Prof. Mihail Barboiu and his co-workers quantify the water permeability and ion transport activity for OH-channels based on vesicles. In the water transport experiments, different concentrations of channel compounds were injected into the vesicle suspension, and after OH-channels were embedded and self-assembled, the vesicles were exposed to an outward directed osmotic pressure gradient. Then, the shrinkage of vesicles was recorded by a Stopped-flow instrument. The hydroxy channels achieve a single-channel permeabilty of 2.33 × 108 water molecules per second, which is within the same order of magnitude as the transport rates for aquaporins. More interestingly, as the concentrations of the injected compounds increased gradually, the water transport rate also increased linearly. Until the concentration over mCLR = 1.5, the permeability of H2, H3 and H4 channels significantly increased by two orders of magnitude, while no significant increase was found in other channels. They put forward that, after reaching a certain stacking density, the channels spontaneously transformed from the originally narrow (2.7 ?) structure to large-scale pores and selectively transport water clusters, and this transport process was verified by molecular dynamics simulations. Ion transport experiments were performed under similar conditions, and the fluorescence traces showed there was no ion or proton transported, which fitted with aquaporins well.
This work demostrates the water transport as water-wire or cluster through OH-channels in the lipid bilayer, and reveals the mechanism of water-wire/cluster transport. Meanwhile, the self-assembled water channel is optimized to achieve complete rejection of ions and protons, which enrich the library of AWCs. Within this context, this discovery of OH-channels opens up new directions and perspectives in AWCs research toward the construction of selective membranes for desalination.
The research progress was recently published in the
Journal of the American Chemical Society and selected as the cover paper. This work was financially supported by NSFC (National Natural Science Foundation of China), CSC (China Scholarship Council), ANR (Agence Nationale de la Recherche WATERCHANNELS) and the Lehn Institute of Functional Materials.
Cite this: L.-B. Huang, A. Hardiagon, I. Kocsis, C.-A. Jegu, M. Deleanu, A. Gilles, A. van der Lee, F. Sterpone, M. Baaden, M. Barboiu.
J. Am. Chem. Soc.
2021, DOI: 10.1021/jacs.0c11952.
Access to this paper:
https://dx.doi.org/10.1021/jacs.0c11952
