Photo-Decaging of A Mitochondria-Localized Iridium(III) Endoperoxide Complex for Two-Photon Photoactivated Therapy under Hypoxia
Source: School of Chemistry
Edited by: Zheng Longfei, Wang Dongmei
Photodynamic therapy (PDT) has become a promising treatment for various diseases, especially oncology. Generally, the photosensitizers (PSs) were delivered to the therapy areas and then given the light of the appropriate wavelength to generate reactive oxygen species (ROS), resulting in apoptosis and necrosis of target cells. Because it has almost no side effects for normal tissue cells and unique clinical advantages. It has been widely used to treat esophageal cancer, non-melanoma skin cancer, cervical cancer and other tumors. The photosensitizer is the core of photodynamic therapy. However, there are still many shortcomings in the clinical application of photosensitizer, such as single type, lack of tumor targeting, poor stability, lack of good water solubility, short-wavelength light excitation, oxygen dependence, etc. Therefore, it is of great significance to develop photosensitizers or photoactivation prodrugs that can generate ROS under hypoxic by the deep tissue penetration depth near-infrared two-photon excitation.
After irradiation, most organic molecules are in the singlet state (S1), and their S1→T1 or T1→S0 transitions are forbidden, resulting in low triplet state excitation efficiency and an unsatisfactory PDT effect. Transition metal complexes, especially ruthenium (II) and iridium (III), have great potential as photosensitizers due to their heavy atomic effect that can increase the intersystem crossing effect and T1 distribution. Prof. Chao Hui's group at Sun Yat-sen University has carried out a series of researches on developing metal photosensitizers and constructed one/two-photon photosensitizers for organelle targeted anti-tumor therapy by using the long excited-state lifetime of metal complexes (Chem. Soc. Rev., 2021, 50, 4185; Coord. Chem. Rev., 2021, 432, 213714; Nat. Chem., 2019, 11, 1041; Nat. Commun., 2020, 11, 3262; Angew. Chem. Int. Ed., 2015, 54, 14049; 2017, 56, 14898; 2019, 58, 14334; 2020, 59, 3315; 2020, 59, 20697; 2021, 60, 4657; PNAS, 2018, 115, 5664; 2019, 116, 20296).
Recently, Prof. Hui Chao (School of Chemistry, Sun Yat-sen University) and Prof. Gilles Gasser (Chimie ParisTech, PSL University, France) reported on novel mitochondria-localized iridium(III) endoperoxide prodrug, which synergistically released upon two-photon light irradiation under hypoxic conditions, highly cytotoxic iridium(III) complex and singlet oxygen as well as generates an alkoxy radical. Due to its independence from oxygen concentration inside cancer cells and its multi-action mechanism, the compound was highly phototoxic in hypoxic tumor cells and 3D multicellular tumor spheroids in the nanomolar range. This study presents the first example of an iridium-based endoperoxide prodrug for synergistic photodynamic therapy/photoactivated chemotherapy under hypoxia. It presents one of the most efficient metal-based PDT PSs working under hypoxic conditions and that can be excited with 2P. Due to a mechanism of action of 2-O-IrAn prodrug involving three independent pathways (1) release of highly cytotoxic iridium(III) complex, (2) generation of singlet oxygen, and (3) generation of an alkoxy radical, extremely high phototoxicity was observed in cancerous cells under normoxic and hypoxic conditions (hypoxia: IC50=60.0 nM, PI=690; normoxia: 51.5 nM, PI=959.1), presenting a novel approach for synergistic photodynamic therapy and photoactivated chemotherapy. Encapsulation within a biotin functionalized polymer into DSPE-PEG-Biotin@2-O-IrAn nanoparticles provides high tumor accumulation and improves the pharmacological properties of the compound. While not showing any side effects within a mouse model, the nanoparticles were found to eradicate a tumor inside an animal model within a single procedure, presenting better therapeutic properties than the clinically approved anticancer drug cisplatin.
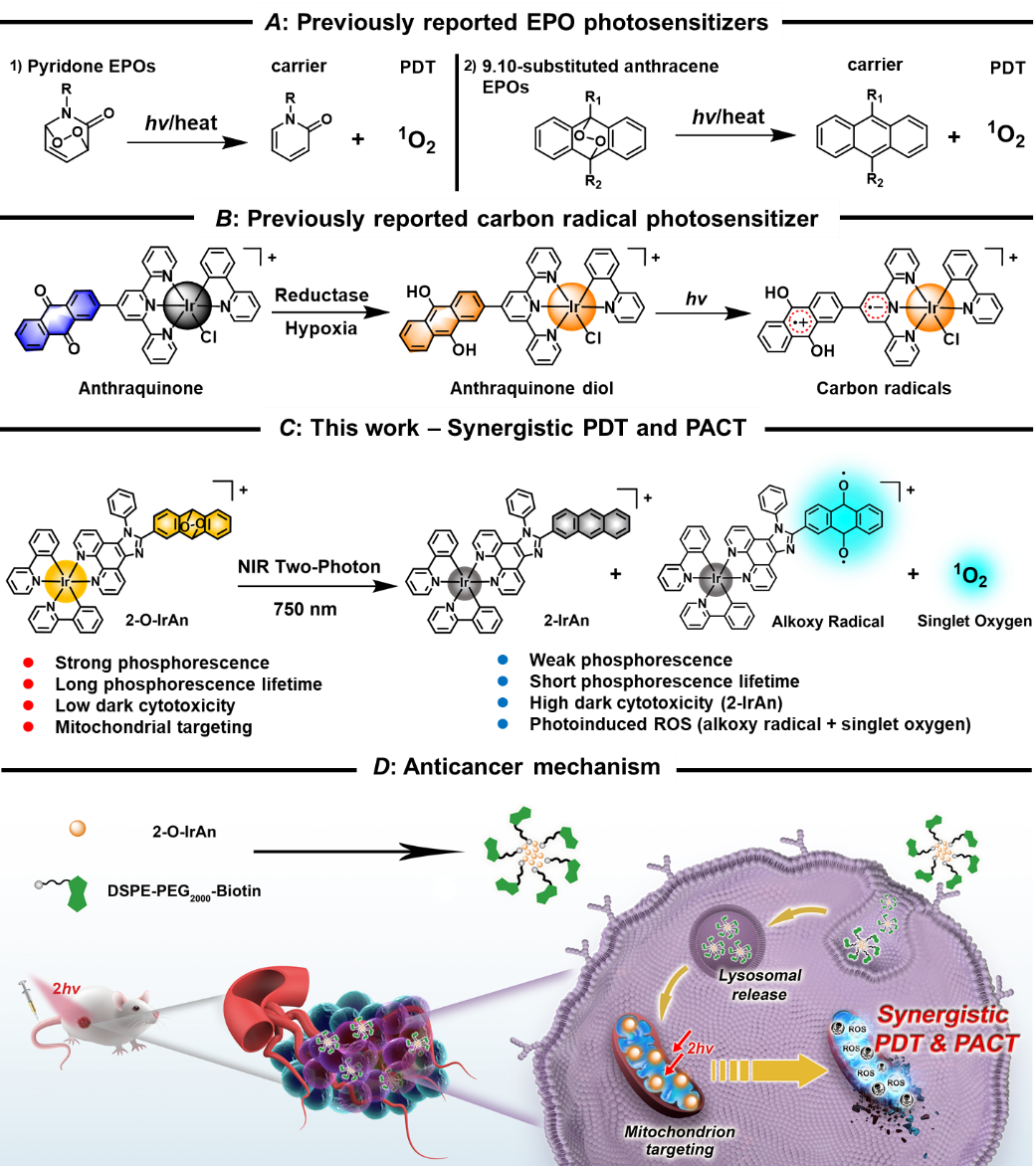
Scheme 1. A) Previously reported EPO photosensitizers, B) Previously reported carbon radical photosensitizers, C/D) Schematic illustration of the mechanism of action of the Ir(III) complex by photodynamic therapy/photoactivated chemotherapy.
This work has recently been published in J. Am. Chem. Soc. entitled: " Photo-Decaging of A Mitochondria-Localized Iridium(III) Endoperoxide Complex for Two-Photon Photoactivated Therapy under Hypoxia". Dr. Shi Kuang and Fangmian Wei from school of Chemistry, Sun Yat-sen University are the co-first authors, Prof. Dr. Hui Chao (from school of Chemistry, Sun Yat-sen University) and Prof. Dr. Gilles Gasser (from Chimie ParisTech, PSL University, France) are the co-corresponding authors.
This work was supported by the National Natural Science Foundation of China (Nos. 22120102002 and 21778079) and the Science and Technology Innovation Program of Hunan Province of China (No. 2021RC5028). This work was also financially supported by an ERC Consolidator Grant PhotoMedMet to G.G. (GA 681679), has received support under the program "Investissements d'Avenir" launched by the French Government and implemented by the ANR with the reference ANR-10-IDEX-0001-02 PSL (G.G.).
Link to the article: https://pubs.acs.org/doi/full/10.1021/jacs.1c13137
