Research News
Neuron | Professor Yamei Tang's team uncovers a chemotactic action of brain resident microglia in recruiting peripheral CD8+ T lymphocytes and driving brain injury
 Updated: Jan 15, 2023
Updated: Jan 15, 2023 Written:
Written:  Edited: Tan Xi, Wang Dongmei
Edited: Tan Xi, Wang Dongmei
Radiation-induced brain injury (RIBI) remains as the most serious medical complication in head and neck cancer patients who received cranial radiotherapy. The clinical manifestations of RIBI include cognitive impairment, epilepsy, and pathological changes such as brain edema and tissue necrosis, which can occur within 3-5 years after the radiotherapy. The progressive development of brain lesions has a negative impact on patients' quality of life, and eventually can result in uncontrolled cerebral herniation and death. Currently, the underlying mechanism of RIBI is still largely unknown, and the limited response rate to available treatments remain a major obstacle to overcome.
On January 5, 2023, Professor Yamei Tang's team from the Brain Research Center and Department of Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University published their work in Neuron entitled "Microglia drive transient insult-induced brain injury by chemotactic recruitment of CD8+ T lymphocytes". The work elucidates the mechanism by which microglia-derived CCL2/CCL8 chemokines recruit peripheral CD8+ T cells to infiltrate brain tissues and cause brain injury in both RIBI and ischemic stroke, providing new pathological insight and a potential therapeutic strategy for both brain diseases.
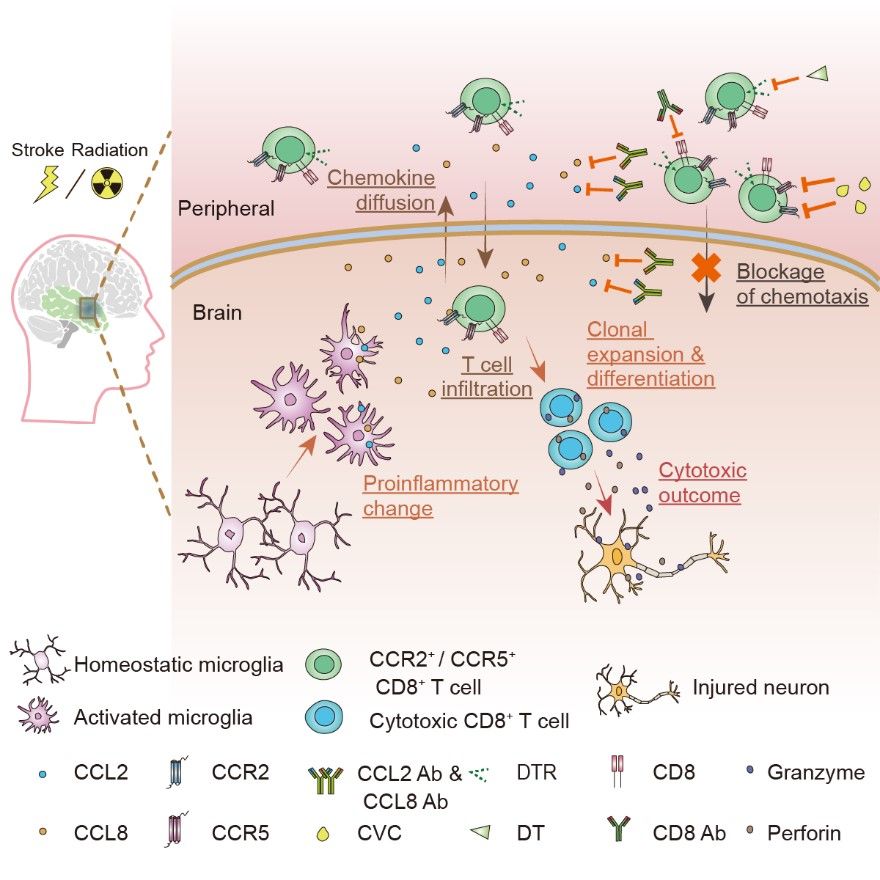
Graphical abstract (Shi, et al, Neuron, 2023)
In recent years, a critical role for T lymphocyte-mediated adaptive immunity in neurological diseases such as Alzheimer's disease and Parkinson's disease has been reported. However, a comprehensive understanding of the interplay between the central nervous system and peripheral T lymphocytes, including chemotactic infiltration and the pathogenic mechanism remain underinvestigated.
In this study, to systematically investigate the cellular and molecular profiles during the pathogenesis of RIBI, the authors applied single-cell RNA sequencing to brain tissues collected from two RIBI patients, and the results identified the infiltration of CD8+ T cells in lesioned brain tissues. Single-cell TCR sequencing was then applied to lesioned brain tissues collected from four additional RIBI patients, which showed significant clonal expansion of infiltrated CD8+ T cells. Using an established RIBI mouse model based on the group's prior study (He et al, FASEB journal, 2020), the authors successfully ablate CD8+ T cells by the use of CD8a-DTR transgenic mice with diphtheria toxin injection, or direct administration of Cd8a neutralizing antibodies in wild-type mice. Both strategies showed robust and systematic depletion of CD8+ T cells, and significantly reduced size of brain lesions in the RIBI mouse model, confirming the pathological role of CD8+ T cells in mediating radiation-induced tissue injury.
Next, the authors investigated the mechanisms of how CD8+ T cells infiltrate brain tissues after cranial irradiation. By applying ligand-receptor pair scanning to the scRNA-seq data, a disease-associated microglial cluster with elevated expression of chemokines such as CCL2 and CCL8 was found to chemotactically interacting with CCR2- and CCR5-expressing CD8+ T cells in the brain. By applying RNAscope in situ hybridization, in vitro cell culture experiments, transgenic mouse models with microglia-specific CCL2 or CCL8 deletion, neutralizing antibodies that blocked CCL2 or CCL8, or small molecule inhibitors of CCL2/CCL8 or CCR2/CCR5, the authors demonstrated that by interfering with the CCL2/CCL8-CCR2/CCR5 chemotactic axis, they could effectively reduce the brain infiltration of CD8+ T cells and significantly attenuate radiation-induced brain lesions.
Infiltration of peripheral CD8+ T cells into the central nervous system has been reported in other neurological diseases. Taking this into account, the authors further examined clinical specimens from ischemic tissues and identified CD8+ T cell infiltration. They next used a mouse model of middle cerebral artery ischemia (MCAO) and identified the same CCL2/CCL8-CCR2/CCR5 chemotactic axis mediated by microglia. Blocking this chemotactic interaction significantly reduced brain infarct volume and CD8+ T cell infiltration in the MCAO mouse model.
Overall, this study demonstrates for the first time that after exposure to radiation or cerebral ischemia insult, microglia secret chemokines CCL2 and CCL8, which then attract peripheral CD8+ T cell infiltration and the release of cytotoxic factors such as perforin and granzyme that cause brain injury. This study also provides a new therapeutic approach to treating RIBI and ischemia by targeting T lymphocyte-mediated adaptive immunity.
Postdoctoral fellows Zhongshan Shi and Pei Yu from the Brain Research Center and Department of Neurology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Associate Professor Wei-Jye Lin and Research Assistant Sitai Chen were the co-first authors of the paper. Professor Yamei Tang was the corresponding author. The study was also supported by Professor Long-Jun Wu from Mayo Clinic and Professor Ho Ko from the Chinese University of Hong Kong. The study was mainly funded by grants from the National Natural Science Foundation of China.
Link to the paper: https://www.cell.com/neuron/fulltext/S0896-6273(22)01082-0
Related News
-
China DailyOct 24, 2024
SYSU researchers used AI to identify new RNA viruses
-
China DailyOct 24, 2024
Sun Yat-sen University Institute to boost East-West exchanges
-
Xinhua NewsOct 23, 2024
SYSU researchers unveil new strategy for cardiac arrest
-
Oct 10, 2024
FLS of SYSU Co-hosted 56th Conference of JEINCS
-
Oct 10, 2024
High-level forum on national strategic needs and high-quality development of foreign language disciplines held at SYSU



