Source: The First Affiliated Hospital of Sun Yat-sen University
Written by: Huan-ling Guo
Edited by: Zheng Longfei, Wang Dongmei
Recently, the interdisciplinary cooperation team of Prof. Xie Xiaoyan from the Department of Ultrasound Medicine of The First Affiliated Hospital of Sun Yat-sen University (FAH-SYSU) and Prof. Shuai Xintao from the School of Materials Science and Engineering of Sun Yat-sen University published their important progress in tumor immunotherapy in Nano Today (IF=20.722). This study provides an approach of synergistic immunotherapy which lowers side effects and mean while improves the prognosis after RFA.
Owing to its minimal invasiveness and short hospital stay, radiofrequency ablation (RFA) as an alternative to surgery has been widely used to treat various solid tumors, including primary or metastatic liver tumors. However, the therapeutic outcome remains limited by the high local tumor progression rate, which is mainly due to the incomplete ablation of target tumors. Different form surgical resection, RFA treatment may result in a burst release of tumor-associated antigens (TAA) that activate adaptive immune response. Nevertheless, based on the clinical practice and literature research around tumor RFA treatment, Prof. Xie Xiaoyan's group found that the anti-tumor immune effect generated after tumor ablation is not strong enough to inhibit the recurrence of residual tumor, and some tumor even progressed after RFA. The relevant mechanism is not yet certain.
In the article, the over-expression of PD-1/PD-L1 pathway induced by RFA was revealed in a incomplete ablative tumor mouse model, which may be a golden point for strengthening the antitumor immunity after RFA treatment via blocking the PD-1/PD-L1 axis. However, a retrospective study illustrated that the PD-1/PD-L1 blockade failed to prolong the progression-free survival of patients after RFA of liver cancers. Further analysis found that the insufficient activation of the STING pathway, resulting in limit infiltration of CD8+T cell in post-RFA residual tumor, is the possible mechanism for the poor response of post-RFA residual tumor to anti-PD-1/PD-L1 monotherapy.
Nevertheless, the clinical application of anti-PD-1/PD-L1 antibodies and STING pathway agonist is still limited by immune-related toxicities caused by non-targeted drug delivery. Additionally, the action sites of STING agonist and αPD-L1 are located on endoplasmic reticulum of immune cells and outer-membrane of cancer cells, respectively. Therefore, a chief challenge for the codelivery of DMXAAst and αPD-L1 acting on different sites is how to spatiotemporally release them in the tumor microenvironment.
To address the challenges mentioned above, the research group developed a nanovesicle capable of releasing the anti-PD-L1 antibody (αPD-L1) and STING agonist inside tumor in a spatiotemporally controlled manner. The nanovesicle entrapping the hydrophilic STING agonist 5,6-dimethylxanthenone-4-acetate sodium salt (DMXAAst) in its lumen and anchoring αPD-L1 via an MMP-2-sensitive peptide linker was coated with PEG layer sheddable in acidic tumor microenvironment. The nanodrug design allowed the PEG coating to block off-target interaction between αPD-L1 and PD-L1-positive normal cells in blood and normal tissues, thereby reducing the immune-related adverse effects (irAEs). A-PD-L1 was firstly released in response to MMP-2 overexpressed in tumor tissue for ICB therapy, which promote the intracellular delivery of DMXAAst to activate STING in DCs. The synergistic effect of αPD-L1, DMXAAst and RFA evoked a robust anti-tumor immunity and long-term immune memory for a potent cancer therapy.
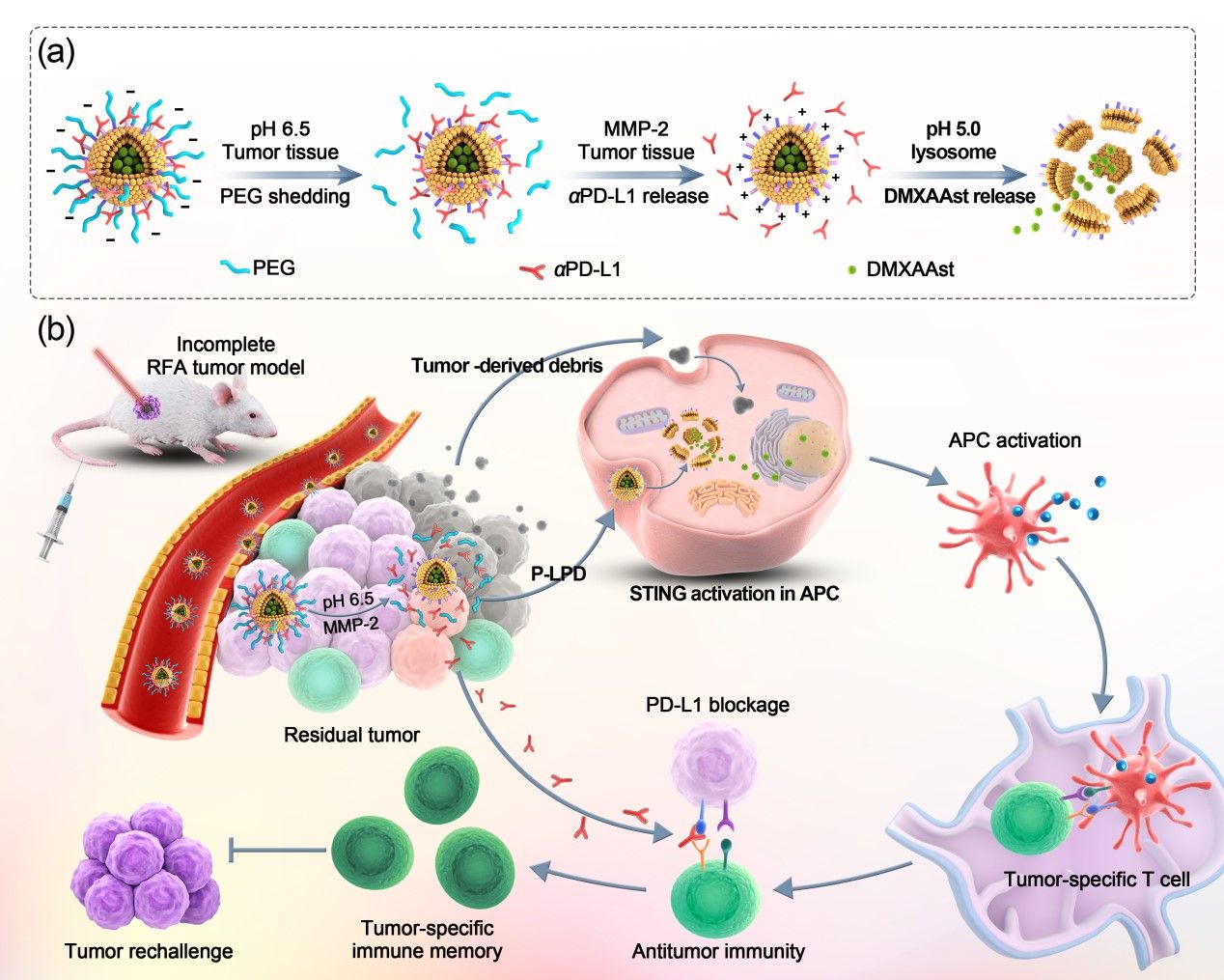
Figure 1. Preparation and anti-tumor performance of the pH and MMP-2 dual-sensitive nanodrug for eliciting robust anti-tumor immune response after RFA Preparation and antitumor performance of the pH and MMP-2 dual-sensitive nanodrug for eliciting robust anti-tumor immune response after RFA.
The research results have been published in Nano Today (IF = 20.722) entitled "Nanodrug shows spatiotemporally controlled release of anti-PD-L1 antibody and STING agonist to effectively inhibit tumor progression after radiofrequency ablation". The co-corresponding authors are Professor Xiao-yan Xie (FAH-SYSU), Professor Xin-tao Shuai (Sun Yat-sen University), Associate Professor Ming Xu (SYSU-FAH) and Associate Researcher Chun-yang Zhang (FAH-SYSU). The co-first author are Postdoc Huan-ling Guo (FAH-SYSU), Assistant Researcher Jin-sheng Huang (The Seventh Affiliated Hospital of Sun Yat-sen University) and Assistant Researcher Yang Tan (FAH-SYSU).
This work was supported by grants from the Major Research plan of the National Natural Science Foundation of China (92059201), the National Natural Science Foundation of China (81530055, 51933011, 31971296, 82071951, 81701696, 82001823, 21905112, 81801703), the Key Areas Research and Development Program of Guangzhou (202007020006), the Key Areas Research and Development Program of Guangdong (2019B020235001).
Link to the paper: https://www.sciencedirect.com/science/article/pii/S1748013222000524



