Source: The First Affiliated Hospital
Edited by: Tan Rongyu, Wang Dongmei
On August 3, 2021, Associate Professor Liu Jianbo from The First Affiliated Hospital of Sun Yat-sen University has published his novel findings in
Chem, one of the top chemical journals in the world. The synthesis of N-trifluoromethyl amides from carboxylic acids was first realized by the traditional amide-bond-forming strategy. The first Affiliated Hospital of Sun Yat-sen University was signature unit of the first author.
Found in biomolecules, pharmaceuticals, and agrochemicals, amide-containing molecules are ubiquitous in nature. The trifluoromethyl group has emerged as an important fluorine-containing functional group frequently found in pharmaceutical compounds. Although elegant methods of synthesizing N–CF3 amides have been previously reported, none have used the simplest retrosynthetic disconnection, namely amide-bond formation through amination of carboxylic acid derivatives. The new approach presented here is based on this disconnection and enables access to a greater variety of N-CF3 amides from readily available carboxylic acids. This strategy can achieve successive desulfurative fluorination and acylation of isothiocyanates in one pot to obtain N–CF3 amides. This approach provides access to a wide range of different N–CF3 amides with various substituents at both the amine and carboxylic acid components. Many of these structures are likely to be inaccessible through previously described methods, especially those products containing alkyl bromide, alpha-heteroaryl, and alpha-carbonyl functional groups. Moreover, the syntheses of N-trifluoromethyl derivatives of several medicinally relevant compounds, such as melatonin and flecainide, were successfully achieved. Given the prevalence of amides and the diversity of available carboxylic acid derivatives and isothiocyanates, the synthesis of N–CF3 amides directly from these components is likely to inspire numerous applications.
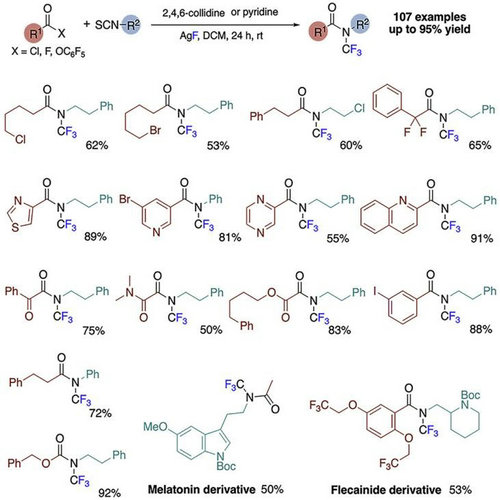
Synthesis of N-trifluoromethyl amides from carboxylic acids
The research was co-authored by Liu Jianbo, Professor F. Dean Toste, University of California, Berkeley, and Professor David M. Wilson, University of California, San Francisco. Professor F. Dean Toste and Professor David M. Wilson are co-corresponding authors of the paper. Part of the research work was completed by relying on the experimental platform of the Department of Nuclear Medicine and the Institute of Precision Medicine of the First Affiliated Hospital, which was supported by the Hundred Talents Program of Sun Yat-sen University.
Link to the research article:
https://www.cell.com/chem/fulltext/S2451-9294(21)00361-2



