Research News
Prof. Yong Zhao’s group at School of Life Sciences reveals a new approach for D-L1-mediated cancer immunotherapy
 Updated: Mar 4, 2019
Updated: Mar 4, 2019 Written:
Written:  Edited:
Edited:
Written by: School of Life Sciences
Edited by: Wang Dongmei
Recently, Prof. Yong Zhao’s group at School of Life Sciences, Sun Yat-sen University, published a research paper entitled “LIN28/let-7/PD-L1 Pathway as a Target for Cancer Immunotherapy” in Cancer Immunology Research (IF=9.2), a renowned journal belonging to the American Association for Cancer Research (AACR). This work revealed the regulatory mechanism of PD-L1 in cancer cells and found a small compound which reactivated T-cell-mediated antitumor immunity, providing a new solution for cancer immunotherapy. The School of Life Sciences and State Key Laboratory of Oncology in South China at Sun Yat-sen university are the first and second communication units, respectively. Yanlian Chen, a postdoctoral fellow of the School of Life Sciences, is the first author of the research. Prof. Yong Zhao is the corresponding author of this paper.
Programmed death ligand-1 (PD-L1) is considered to be an immune-check-point protein. However, it is commonly overexpressed on cancer cell surface and interacts with programmed cell death protein-1 (PD-1) on T-cell surface, most likely as a mechanism to promote T-cell tolerance and escape immune surveillance. Nowadays, cancer immunotherapy uses monoclonal antibodies against PD-1 or PD-L1 to block the PD-1/PD-L1 signaling pathway, thereby reactivating T cell and killing cancer cells. However, the response to antibody treatment was observed in fewer than 40% of patients, which could reflect the individual variation in PD-L1 expression and the complexity of tumor microenvironment. Thus, a new powerful approach is in urgent need.
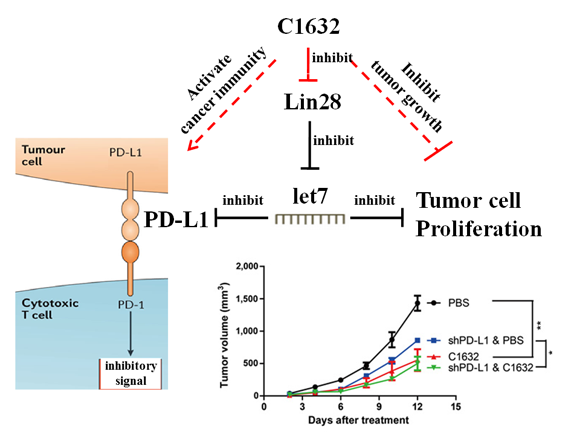
Schematic diagram showing that C1632 reactivates T-cell-mediated cancer immunity and suppresses tumor growth
Prof. Yong Zhao’s group reported that the expression of PD-L1 was regulated by LIN28/let-7, a signaling pathway widely exists in cancer cells. Therefore, inhibition of LIN28 sufficiently downregulated amount of PD-L1 in cancer cells. Moreover, they found treatment with a LIN28 inhibitor, the small compound C1632, suppressed PD-L1 expression on cancer cell surface, leading to prevention of cancer immune evasion and reactivation of T-cell-mediated antitumor immunity both in vitro and in vivo. In addition, C1632 also displayed the capacity to inhibit cancer cell proliferation. Collectively, the small compound C1632 has dual function in cancer therapy (as shown in the figure above).The identification of LIN28/let-7/PD-L1 pathway and C1632 provides a new approach for PD-L1-mediated immunotherapeutics and a promising agent for chemotherapy and immunotherapy. C1632 has high water solubility, good permeability, high thermal stability and low cytotoxicity. It works in multiple cancer cell types, including breast cancer cells, cervical cancer cells, osteosarcoma and lung cancer cells, which is expected to further expand the scope of application of PD-1/PD-L1 targeting immunotherapeutics and improve antitumor efficacy, implying important research and clinical application value. It is told that they have already applied international and domestic patents to protect “the regulatory mechanism of PD-L1 by LIN28/let-7” and “application of small compound C1632 in cancer immunotherapy”.
Link to the paper: http://cancerimmunolres.aacrjournals.org/cgi/content/abstract/2326-6066.CIR-18-0331



